Affiliate Disclaimer
Some links in this article are affiliate links. We may earn a small commission if you make a purchase through these links, at no extra cost to you. We only recommend products we find useful to our readersType 1 Diabetes is an autoimmune chronic disease, which is characterized by the immune system attacking beta cells in the pancreas, known to produce insulin. Unlike Type 2 Diabetes, whose causes have increasingly hinged on lifestyle factors, T1D mainly results from an autoimmune response. It’s crucial to identify triggers of this immune reaction for early detection and possibly prevention. This article explains the genetic and environmental factors involved in causing the onset of T1D, shedding light on how the disease evolves in genetically predisposed individuals.
Genetic Predisposition

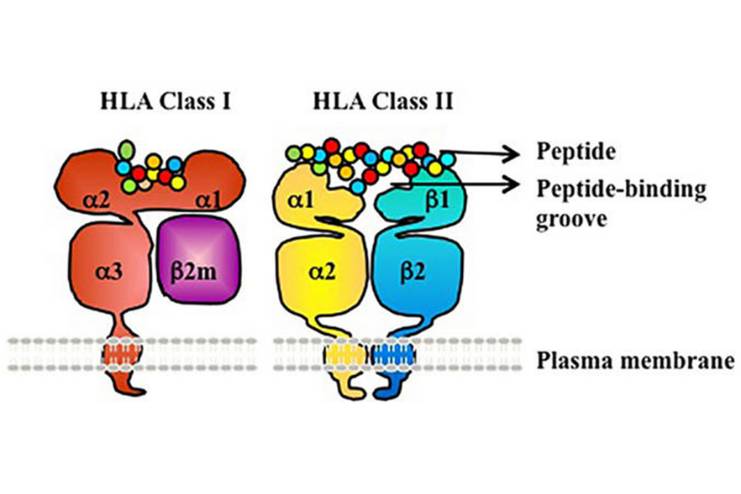
Type 1 diabetes mellitus is basically an autoimmune disease characterized by cell-mediated destruction of the insulin-producing beta cells in the pancreas. Genetic predisposition accounts for most of the susceptibility towards T1DM. About nearly 50% of the factors are hereditary. The HLA complex, especially the class II genes like HLA-DR and HLA-DQ, are markedly associated with T1DM. Some HLA haplotypes, such as DR4-DQ8 and DR3-DQ2, conferred a very high risk of T1DM. The highest susceptibility was seen in those persons carrying both haplotypes.
GWAS have identified over 50 loci that are not HLA and contribute to the risk of T1DM. Prominent genes include the INS gene (insulin gene) and PTPN22 (protein tyrosine phosphatase non-receptor type 22), with functions in the modulation of immune responses and the production of insulin. The class I alleles of the INS VNTR gene control insulin expression and increase the risk of T1DM.
All of these mean that genetic factors play a central role in the explanation of T1DM, and much remains to be learned about just how these genes interact to influence the development of disease.
Environmental Factors

The environmental factors play a more significant role in the presentation of T1D, and this usually happens among genetically predisposed individuals. Environmental triggers, rather than predisposing genes, are what set off or accelerate the autoimmune cascade, which leads to the destruction of the insulin-producing beta cells within the pancreas.
Autoimmune Mechanism
The autoimmune nature of T1D involves various immune cells and pathways:
- Effector T Cells (Teff) and Cytokines: Th1 cells, that produce IFNγ, are promoting pathology within the islets by recruiting more immune cells and exacerbating local inflammation. There is infiltration of islets by Th17, but their roles less well defined. IL-21, mainly produced by follicular helper T cells, is involved in B-cell development and increases the survival of CD8+ Teff, both leading to islet destruction.
- Neoautoantigens: Inflammatory processes result in the production of neoautoantigens by post-translational modifications, which are recognized by pathogenic CD4+ and CD8+ T cells, hence fueling the immune response further.
- Treg Dysregulation: Defects in Foxp3+ regulatory T cells (Treg), necessary for immune tolerance homeostasis, contribute to T1D. Foxp3+ Treg deficiencies allow pathogenic Teff to expand with unchecked license, thereby worsening the autoimmune response.
- β Cell-Intrinsic Factors: β cells can produce chemokines and cytokines (for example, CXCL10, CCL2, IL-1β) that modulate immune cell migration and inflammation. Some of the β cells may adapt to inflammatory conditions by down-modulating autoantigens and increasing the resistance against immunity. These may provide potential therapeutic insights into β cell replacement therapies.
These insights into the immunity pathways that are apparently involved in T1D highlight the complexity of interaction between immune cells and β cells, thus pointing to possible therapeutic targets for modulation of the immune system and preservation of β cells.
Immune System Dysregulation
T1D is marked by immune system dysregulation:
- Genetic Susceptibility: The most significant contribution in genetic susceptibility to T1D is that of the human leukocyte antigen (HLA) region on chromosome 6. Specifically, certain HLA class II alleles, including HLA-DR and HLA-DQ, have a strong association with enhanced predisposition to autoimmune responses resulting in T1D.
- Breakdown of Immune Tolerance: Failure of immune tolerance leads to the activation of autoreactive T cells targeting the pancreatic islet cells, mainly the beta cells, which it later marks for destruction.
- Key Players Involved in Dysregulation: CD4+ and CD8+ T cells are the primary players in cell-mediated destruction of beta cells. CD4+ T cells assist in the activation of other immune cells, whereas CD8+ T cells destroy the beta cells with the help of autoantigens. B-cells produce autoantibodies against the components of the beta cells like insulin and GAD65. Generally, regulatory T cells (Tregs) which help to dampen excessive immune responses are defective in T1D patients as they cannot regulate autoreactive immune cells.
- Inflammatory Cytokines and Chemokines: The existence of such proinflammatory cytokines like IL-1β, TNF-α, and IFN-γ also creates an inflammatory environment within the pancreatic islets, with more beta cell stress and death. More immune cells are recruited through chemokines such as CXCL10 to the pancreas for strengthening the attack.
- Changes in DNA Methylation and Histone Modifications: These contribute to changes in the expression of genes involved in immune regulation, potentially leading to inefficient T1D immunity tolerance mechanisms.
Autoantibodies as Markers for Immune Dysregulation Autoantibodies, such as anti-insulin, anti-GAD, and anti-IA2, are thus a marker for immune dysregulation in T1D. Autoantibodies develop years before clinical symptoms have become apparent, so the attack is always ongoing well before the loss of beta cell function.
Environmental factors, including viral infections or gut microbiota imbalance, can also trigger the occurrence of immune dysregulation and therefore accelerate the autoimmune process.
Conclusion
There is an imperative need for progress towards early detection, intervention, and even prevention of Type 1 Diabetes (T1D) based on new understanding of the autoimmune triggers for this chronic disorder. The interplay between predisposing genetic components and environmental factors places this contribution toward the pathogenesis of T1D within more complex mechanisms. The roles of the immune cells, genetic variants, and environmental triggers are going to be unraveled so that new therapeutic targets become open in attempts at preserving the function of beta cells and restoring tolerance of the immune response. More exploration of these characteristics of T1D gives not only deeper insight into this disease but also opens new horizons for innovation in treatment modalities that may improve the quality of life in those with this disease. The more that is grasped of the immunological and genetic context within which T1D occurs, the closer we become to revolutionizing the way we handle and prevent this amazingly challenging autoimmune condition.
In this Article